12399456https://www.upal.com.my/zh/feeds/jobFeedKwongwahArray
(
[0] => HTTP/1.1 200 OK
[1] => Date: Sun, 15 Feb 2026 12:24:15 GMT
[2] => Content-Type: text/xml; charset=utf-8
[3] => Connection: close
[4] => Cache-Control: no-cache, private
[5] => Cache-Control: no-cache, no-store, must-revalidate
[6] => Set-Cookie: UpalLiveSession=eyJpdiI6InpcL0dtaTRUUFZyTW5zd0VHZXExaEJnPT0iLCJ2YWx1ZSI6InZPRlRLUkRDdVhXaFZcL0NLZVI5dE5TTTFvbDN4S3M0OU1TRGUxZUZiVFhwdnlGRXNOaDVYenFsQ2xIMlUyOVZtXC9VNXNqdWp5MGdiRk9PT1o0V2lQakE9PSIsIm1hYyI6ImE1YzAyNjNiODZiOGNlNWQ3NmJjYWI1ODU0YTMzNzkxMTdhNTVjMWNlNWI2OTk3OGI5NjEzYTI3NzAyZTJkOGYifQ%3D%3D; expires=Wed, 14-Feb-2029 12:24:15 GMT; Max-Age=94608000; path=/; HttpOnly
[7] => x-frame-options: SAMEORIGIN
[8] => x-content-type-options: nosniff
[9] => x-xss-protection: 1; mode=block
[10] => referrer-policy: strict-origin-when-cross-origin
[11] => permissions-policy: geolocation=(), microphone=(), camera=()
[12] => content-security-policy: default-src * 'unsafe-inline' 'unsafe-eval' data: blob:; script-src * 'unsafe-inline' 'unsafe-eval' data: blob:; style-src * 'unsafe-inline' data:; img-src * data: blob:; font-src * data:; connect-src *; frame-src *; object-src *; media-src *; child-src *;
[13] => Server: cloudflare
[14] => x-robots-tag: noindex, nofollow, nosnippet, noarchive
[15] => strict-transport-security: max-age=31536000; includeSubDomains; preload
[16] => expires: Mon, 16 Feb 2026 12:24:15 GMT
[17] => vary: Accept-Encoding
[18] => Report-To: {"group":"cf-nel","max_age":604800,"endpoints":[{"url":"https://a.nel.cloudflare.com/report/v4?s=IhR4nmkwqhMDHMop9DvOWoCuJSkSCxtnfyfLsmwulv%2B9RlfhoTmeQ%2Fv6axLyhLc1Lb7rgPVgGQs6pXCqluEHMJeDbszYV2hgkN9PiiVbKA%3D%3D"}]}
[19] => cf-cache-status: DYNAMIC
[20] => Nel: {"report_to":"cf-nel","success_fraction":0.0,"max_age":604800}
[21] => Speculation-Rules: "/cdn-cgi/speculation"
[22] => Server-Timing: cfCacheStatus;desc="DYNAMIC"
[23] => Server-Timing: cfEdge;dur=28,cfOrigin;dur=133
[24] => CF-RAY: 9ce4c7f6d9a0fdf3-SIN
[25] => alt-svc: h3=":443"; ma=86400
)
789
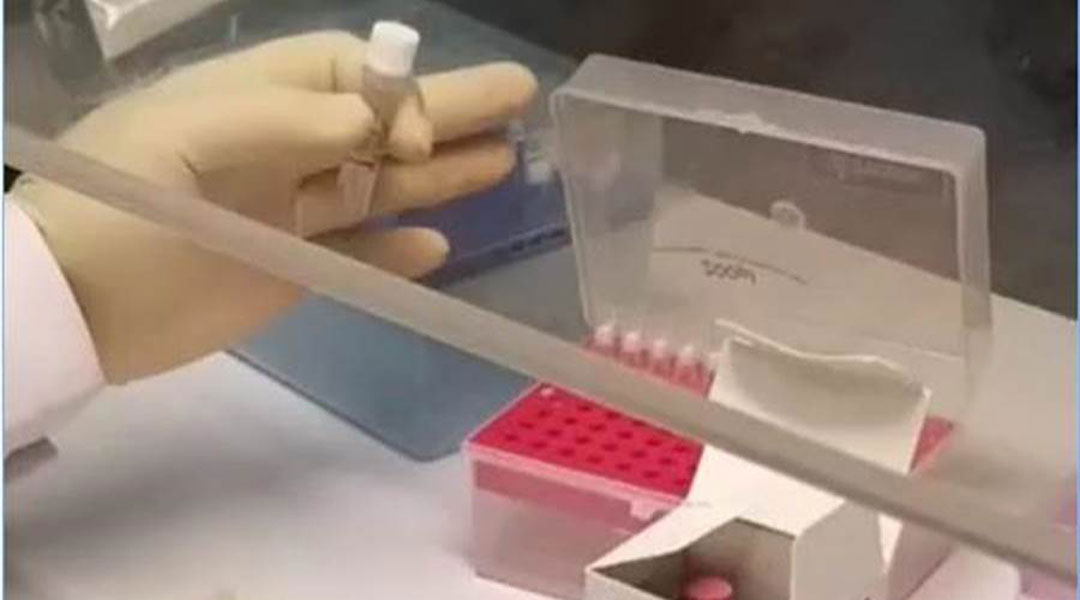
中国新型冠状病毒核酸检测试剂盒通过官方审批,30分钟可得结果。
中国研发的新型冠状病毒核酸检测试剂盒,已于28日通过中国国家药品监督管理局审批,获得医疗器械注册证书。
这个新测试剂盒宣称可在30分钟内获得结果,大幅缩短检测时间,可以让疫情疑似病例,尤其是没有病症的隐性感染者得到快速分流,有效防止疫情扩散。
这项产品由中国圣湘生物科技公司推出,中国体外检测诊断领域专家戴立忠主导的应急技术攻关小组研发,采用“RNA一步法”技术,适用于不同场景,操作简单,检测耗时短,再搭配圣湘生物自主研发的全自动核酸提取仪、移动分子诊断平台,能实现1人1机1日检测样本量达1000人份以上的高通量检测,最快可在30分钟出检测结果,实现现场即时检测。
找工作, 就找这里!

› 立即申请
- GMBB Part Timer
- Event
- Kuala Lumpur
-

MYR 110.00 /Month

› 立即申请
- Social Media Marketing Executive
- Advertising & Marketing
- Kuala Lumpur
-

MYR 6K /Month

› 立即申请
- PHP Software Developer
- Information Technology
- Wilayah Persekutuan
-

MYR 6K /Month

› 立即申请
- DevOps Software Engineer
- Information Technology
- Kuala Lumpur
-

MYR 6.5K /Month

› 立即申请
- Java Software Engineer
- Information Technology
- Kuala Lumpur
-

MYR 10K /Month

› 立即申请
- Admin cum Customer Service
- Engineering
- Bayan Lepas
-

MYR 3K /Month

› 立即申请
- HR Recruiter (Internship)
- Human Resources
- Kuala Lumpur
-

MYR 850.00 /Month

› 立即申请
- PHP Software Engineer
- Engineering
- Kuala Lumpur
-

MYR 4K /Month

› 立即申请
- PHP Software Engineer (Internship)
- Engineering
- Kuala Lumpur
-

MYR 800.00 /Month

› 立即申请
- Graphic Design + Marketing (Internship)
- Advertising & Marketing
- Kuala Lumpur
-

MYR 800.00 /Month

› 立即申请
- PHP Web Developer (Internship)
- Engineering
- Kuala Lumpur
-

MYR 800.00 /Month

› 立即申请
- Multimedia - Video & Marketing (Internship)
- Advertising & Marketing
- Kuala Lumpur
-

MYR 800.00 /Month

› 立即申请
- PHP Web Developer
- Engineering
- Kuala Lumpur
-

MYR 4K /Month

› 立即申请
- Software Developer
- Information Technology
- Kuala Lumpur
-

MYR 4K /Month

› 立即申请
- 软件测试与客户支持专员 Software Testing & Customer Support Specialist
- Information Technology
- Kuala Lumpur
-

MYR 3K /Month
//
//
//
//
















